Biocompatibility Testing
Ensuring safety and efficacy of medical devices on patients with biocompatibility testing
Biocompatibility is a critical concept in the development and evaluation of medical devices and materials intended for use within the human body. It refers to the ability of a material to perform with an appropriate host response in a specific application. Ensuring biocompatibility is essential because it determines how well a material interacts with the body's biological systems without causing adverse reactions such as toxicity, inflammation, or immunological rejection. This is especially crucial for implants, prosthetics, and other medical devices that come into direct contact with tissues, blood, or bodily fluids.
The principle of biocompatibility testing involves a comprehensive evaluation of materials through a series of standardized tests, which assess various aspects such as cytotoxicity, sensitization, irritation, and systemic toxicity. These tests are designed to identify potential risks and ensure that materials do not release harmful substances or degrade in a way that could compromise patient safety. They are used globally by manufacturers, regulatory agencies, and testing laboratories to validate the biocompatibility of medical products.
The concept of biocompatibility extends beyond just the absence of negative reactions; it also encompasses the material's ability to integrate and function as intended within the biological environment. This involves considering factors such as the material's mechanical properties, its interaction with surrounding tissues, and its long-term stability and performance. Understanding and ensuring biocompatibility is fundamental to advancing medical technology and improving the quality and safety of healthcare solutions.
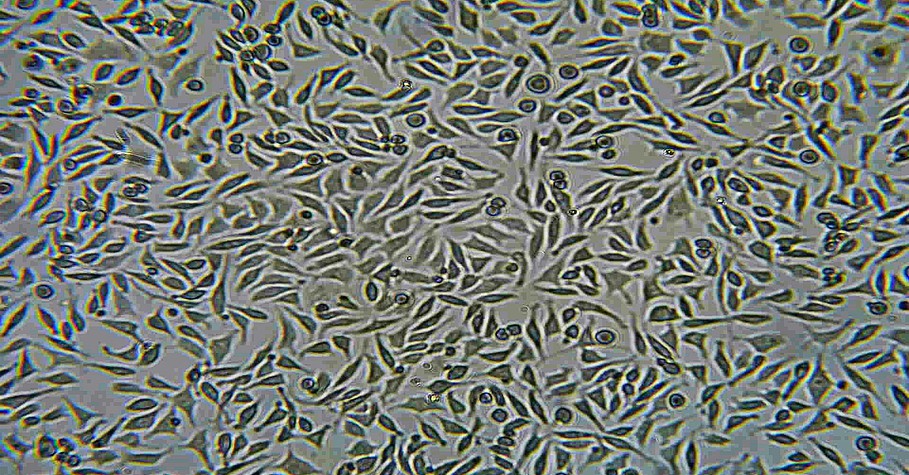
ISO 10993-5 Tests for in-vitro cytotoxicity
ISO 10993-5 is an international standard that specifies test for cytotoxicity, which is the ability of a material to cause harmful effect on cells. This standard outlines methods to evaluate whether medical devices and materials induce cytotoxic reactions, which could potentially harm patients. Testing involves exposing cultured cells to extracts of the material being evaluated and observing any adverse effect such as cell death or interference with cellular function. Compliance with ISO 10993-5 helps ensure that medical devices and materials are safe for their intended use, contributing to the overall safety and efficacy of healthcare products.
OECD 439* Tests for in-vitro skin irritation - Reconstructed Human Epidermis (RHE)
OECD 439 in-vitro skin irritation using Reconstructed Human Epidermis (RHE) is a standardized test method designed to evaluate the skin irritation potential of chemicals and products without using animal testing. These RHE models are cultured from human keratinocytes and reproduce key features of the epidermis, including the stratum corneum (outermost protective barrier). This mimics the structure and function of the human skin's outer layer, allowing for a realistic assessment of how a substance might affect human skin.
By applying the test substance to the RHE model and measuring cell viability, the method determines whether or not the product is a skin irritant. If the viability falls below a certain threshold, the substance is deemed to be a skin irritant.
OECD 439 is internationally recognized and accepted by regulatory bodies. It provides a robust and reproducible method for safety assessment and offers a reliable and ethical alternative to traditional animal testing, ensuring that products meet the necessary safety standards before they reach the market.
OECD 431* Tests for in-vitro skin corrosion - Reconstructed Human Epidermis (RHE)
OECD 431 in-vitro skin corrosion using Reconstructed Human Epidermis (RHE) is an animal-free alternative for testing skin corrosion. This standard method uses a three-dimensional model of human epidermis created from cultured keratinocytes, which mimics the key features of human skin's outer layers, simulating real-life exposure.
The test exposes the model to a substance for a set period and measures tissue viability. If cell viability falls below a set threshold, it indicates the substance has corrosive potential.
OECD 431 provides a reliable, ethical and reproducible way to assess skin corrosion without animal testing, in compliance with safety regulations.
* denotes tests not covered under our ISO/IEC 17025 accreditation.

